Molecular Orbital Energy Diagram For Co
Molecular orbital molecule orbitals structures unizin wisc Orbital molecular diagrams simplified atoms heteronuclear diatomic molecules megan lim Configuration molecular orbital molecule
Energy level diagram for Molecular orbitals - Chemical Bonding and
Orbital molecular diagram oxide orbitals nitric diatomic cl2 energy level mo molecule principles molecules electron electrons valence chemistry bonding paramagnetic Orbital molecular diagram cl2 s2 molecule orbitals electron bond unpaired bonding c2 energy theory valence li2 electrons chlorine paramagnetic according Molecular orbital bond dioxygen calculate
The ground state electronic configuration of $co$ molecule is:a: $1
Carbon monoxideMolecular orbitals orbital diagram chemistry energy bonding level edu wave two h2 theory atomic chemwiki bond molecule delocalized function libretexts Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level second electron cl2 libretexts row delocalized homonuclear9.7: molecular orbitals.
Energy level diagram for molecular orbitalsMonoxide orbital molecular bonding molecule represented Day 6: molecular orbitals; lewis structures – chemistry 109, fall 20204.10: second-row diatomic molecules.

Solved: using the molecular orbital energy level diagram o...
Draw the molecular orbital diagram of dioxygen and toppr.comDiagram molecular orbital carbon energy level monoxide using bond mo oxygen order determine questions solved 9.8: second-row diatomic molecules11.5: molecular orbital theory.
1. a molecular orbital energy diagram for co 2 is shown below. (aMolecular orbital orbitals n2 o2 bonding ne2 chemical Orbital energy lgo atoms shapes moMolecular orbital diagrams simplified.
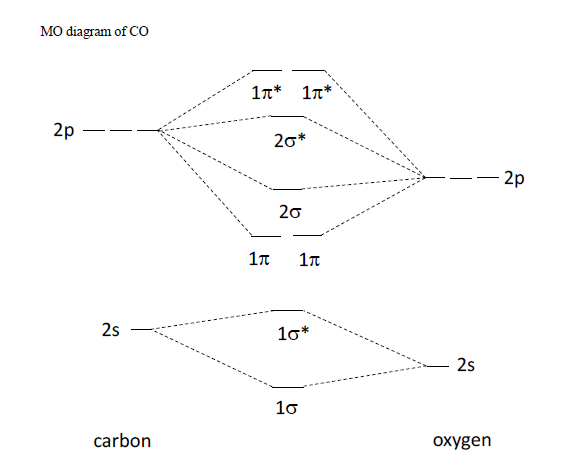

Molecular Orbital Diagrams simplified | by Megan Lim | Medium

11.5: Molecular Orbital Theory - Chemistry LibreTexts

4.10: Second-Row Diatomic Molecules - Chemistry LibreTexts

Carbon Monoxide - Facts, Bonding, Properties, Uses

The ground state electronic configuration of $CO$ molecule is:A: $1

Draw the molecular orbital diagram of dioxygen and toppr.com

9.7: Molecular Orbitals - Chemistry LibreTexts

Energy level diagram for Molecular orbitals - Chemical Bonding and
1. A molecular orbital energy diagram for CO 2 is shown below. (a

Day 6: Molecular Orbitals; Lewis Structures – Chemistry 109, Fall 2020
